Orario: Popolarità:0volte
BET Surface Area Analysis Method
The BET (Brunauer-Emmett-Teller) surface area analysis method derives its name from the BET theory developed by the three scientists after whom it's named. This theory established the multimolecular adsorption equation, forming the theoretical basis for particle surface adsorption science and finding widespread application in research and instrumental data processing related to particle adsorption properties.
Common Instrument Models
STD-ASAP Series

Autosorb Series

Fundamental Principles
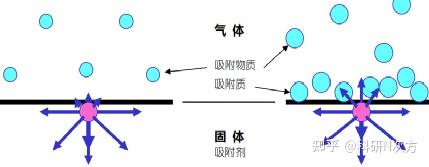
Gaseous atoms and molecules exhibit free motion, while solid atoms remain in fixed positions due to electrostatic forces from neighboring atoms. However, surface atoms experience imbalanced forces as they have fewer adjacent atoms compared to inner atoms. This force imbalance creates surface energy, leading surface atoms to adsorb gas molecules from the surrounding atmosphere to compensate for the electrostatic imbalance. Unlike liquid surface molecules that can move, solid surface atoms remain stationary, resulting in higher surface energy for most solids compared to liquids.
Six Adsorption Isotherm Types
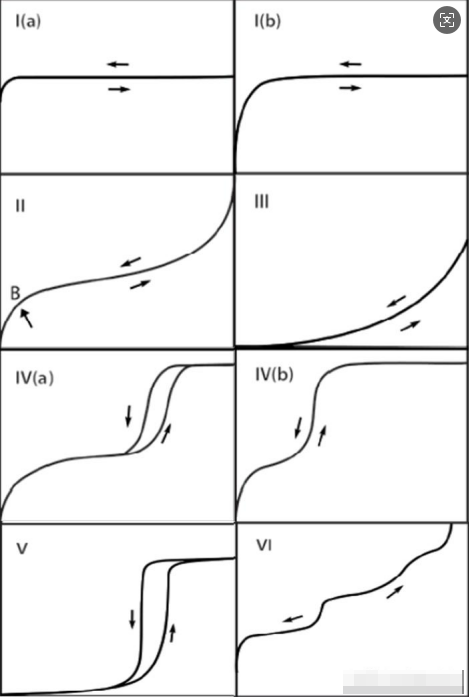
Type I: Bends toward the P/P₀ axis with a horizontal or near-horizontal plateau, indicating adsorption saturation limited by micropore volume rather than internal surface area. Rapid adsorption at very low P/P₀ reflects micropore filling (pore width <1nm for nitrogen at 77K). At saturation pressure (P/P₀>0.99), adsorbate condensation may cause upward curvature. Microporous materials exhibit this isotherm:
Type II: Reversible isotherm for non-porous or macroporous materials, showing unrestricted monolayer-multilayer adsorption. A sharp inflection point (B) indicates monolayer completion, while gradual bending suggests overlapping monolayer coverage and multilayer onset. No plateau at P/P₀=1 implies unlimited multilayer growth.
Type III: Observed in non-porous/macroporous solids with weak adsorbent-adsorbate interactions. No distinct monolayer formation (no point B), with molecules clustering at most attractive surface sites. Limited adsorption at saturation pressure.
Type IV: Characteristic of mesoporous materials (e.g., oxide gels, industrial adsorbents). Initial monolayer-multilayer adsorption follows Type II, followed by capillary condensation in mesopores. Features a final adsorption plateau (IVa with hysteresis) or fully reversible desorption (IVb for narrow mesopores <4nm). Hysteresis originates from capillary condensation at pressures below bulk liquid saturation pressure (P₀).
Type V: Similar to Type III at low P/P₀ but shows inflection at higher pressures from pore filling by molecular clusters. Observed in hydrophobic micro/mesoporous materials during water adsorption.
Type VI: Stepwise reversible adsorption from highly uniform non-porous surfaces, with each step corresponding to completed monolayer coverage. Step height indicates layer capacity, while sharpness depends on system and temperature. Incomplete forms observed for nitrogen at 77K.
Hysteresis Loop Types
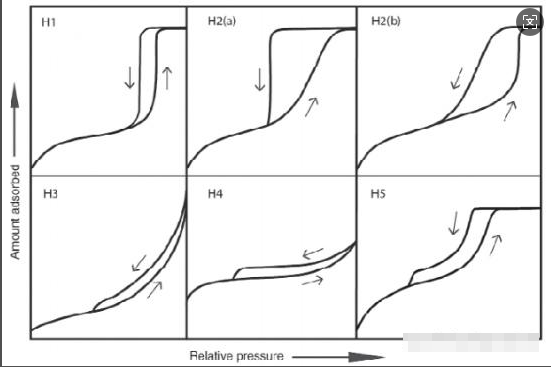
While physical adsorption is theoretically reversible, adsorption-desorption isotherms often diverge in porous materials due to capillary condensation. This phenomenon creates hysteresis loops classified as:
H1: Narrow cylindrical mesopores with minimal network effects, featuring steep desorption branches from delayed condensation. Also observed in "ink-bottle" pores with neck size distributions matching pore dimensions.
H2: Complex pore structures with significant network effects:
H3: Slit-shaped meso/macropores in layered aggregates, with adsorption branches resembling Type II and desorption starting at cavitation-induced pressures. Typical of non-rigid aggregates with incomplete pore filling.
H4: Combines Type I and II adsorption features with significant micropore filling at low P/P₀. Observed in zeolites, mesoporous zeolites, and micro-mesoporous carbons with narrow slit pores.
H5: Rare type found in partially blocked mesoporous materials with both open and occluded pore structures. Desorption branches drop sharply in narrow P/P₀ ranges (e.g., 0.4-0.5 for nitrogen at 77K), a feature shared with H3 and H4 loops.
Telefono aziendale
+86-21-6420 0566
Orario di lavoro
Da lunedì a venerdì
Telefono cellulare:
13816217984
Posta elettronica:
info@qinsun-lab.com
